Introduction
Insert intro narrative or video.
Objectives
By the end of this lesson students should be able to:
- Explain the role of Extractive Metallurgy and its sub disciplines in mining industry.
- Explain mineral processing and processes involved.
- Distinguish between ore, mineral and rocks.
- List different physical properties of ore and how they are exploited in beneficiation process.
- Develop an understanding how beneficiation process works based on a typical example flowsheet of various ore.
Reading
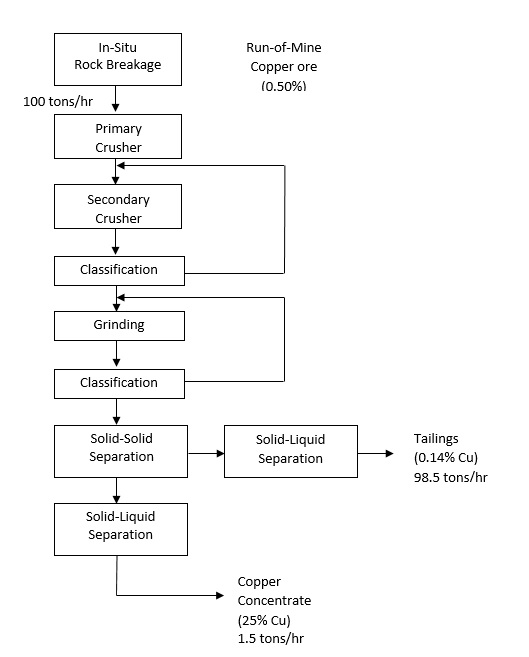
The economical production of metals and non-metals has never been as important and as difficult as it is today. In the twentieth century, annual copper production increased by a factor of 250 to an amount exceeding 8 million tons per year. A greater increase was realized by aluminum with similar trends also observed for other metals such as lead, zinc and tin. Occurring simultaneously with the increase in demand of most metals, industrial minerals and fuels, the quality of the ore reserves have depreciated substantially.


[image (135-1-2.1]

[image 135.1.2.2]

[image 135-1-2.3]

[image 135-1-2.4]
Today’s ore reserves are lower in grade and the minerals are more finely disseminated, thereby making minerals processing of the material more complex and costly. Finely disseminated ores require substantial comminution costs to liberate the valuable minerals. In 1986, it was estimated that 35% of selling price for copper was associated with the crushing and grinding of host ore.

[image 135-1-3]
Extractive Metallurgy
Sub-disciplines:
- Minerals Processing
- Hydrometallurgy
- Pyrometallurgy
- Electrometallurgy
Mineral Processing
Distinct objectives include:
- Production of a saleable material having a specified particle size distribution (e.g. aggregate industry).
- Liberation of components for subsequent processing (e.g. exposing surfaces for leaching processes).
- Generation of a material having a specified composition, e.g.,
- Elimination of components that would hinder the efficiency of downstream processes.
- Elimination of components that would limit the feasibility of using the materials as a saleable product.
- Objective ‘c’ involving the production of a final concentrate tends to be the most complex and difficult forms of mineral processing since it involves several distinctly different operations, i.e.,
- Liberation (crushing and grinding);
- Particle Size Control (screening and classification);
- Composition Control (solid-solid separations);
- Product and Tailings Dewatering (solid-liquid separations).
Aggregate
In the definition of aggregate the term “rock” excludes metallic ores and the non-metallic ores: barite, coal, diamond, graphite, gypsum, kaolin, magnesite, mica, salt and talc.
The production of aggregates is relatively simple. The process typically involves a series of crushers and screens to produce material having various particle sizes.
Importance of Aggregates
- Aggregates touch our lives everyday, from the driveway to the workplace. We drive, sit, stand and walk on aggregates.
- Many products that enrich our daily lives contain aggregates. They are found in paint, paper, plastics and glass. In powder form, aggregates are used as mineral
supplements for agriculture, medicines and household products. - Aggregates are also used to protect the environment by controlling soil erosion, assisting in water purification and reducing sulfur dioxide emissions generated by power plants.
Aggregate Production
At the beginning of the 20th century, production of aggregates in the United States was minimal and its uses limited. Today, aggregates are produced in every state, and aggregates production tonnage ranks first in the non-fuel minerals industry.
More than two billion tons of aggregates are used annually in the United States. This equals ten tons of aggregates for every American!

[image 135-1-5]

[image 132-1-5.2]
Aggregate Preparation Circuits
- Grizzly Screens: Removes fines to bypass primary crushers.
- Crushers: Reduces the size of material.
- Screens: Separation aggregates into various sizes.

[image 135-1-6]
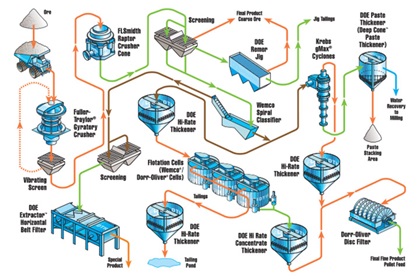
Iron Ore Processing
The mineral types are:
- Hemattite (Fe2O3)
- Magnetite (Fe3O4)
Typical gangue material is silica
In some cases, the iron ore deposit is sufficiently rich and thus no physical processing is required.
Where processing is required, separation processes typically include:
- Density-based separators
- Magnetic separators
- Froth flotation

[image 135-1-9]
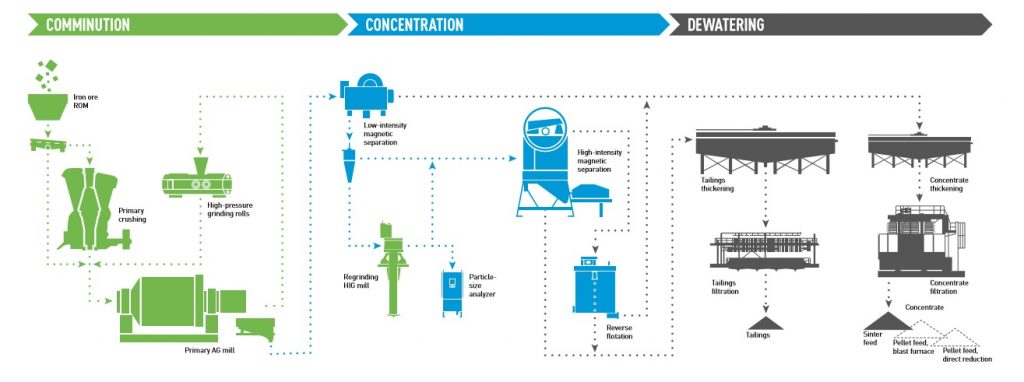
Iron Processing Circuits
- Grinding (9’- powder-fine)
- Primary Mill Grinding
- Pebble Mill Grindin1g
- Concentrating:
- Deslime thickeners
- Magnetic separator
- Vacuum disc filters
- Producing Pellets:
- Balling: The powdery iron ore concentrate is mixed with a small amount of a clay binder called “bentonite’ and rolled into marble-sized pellets.
- Rotary Kiln: Heat-hardening of pellets at temperatures as high as 2,400 °F.
[image 135-1-10]

[image 135-1-13]
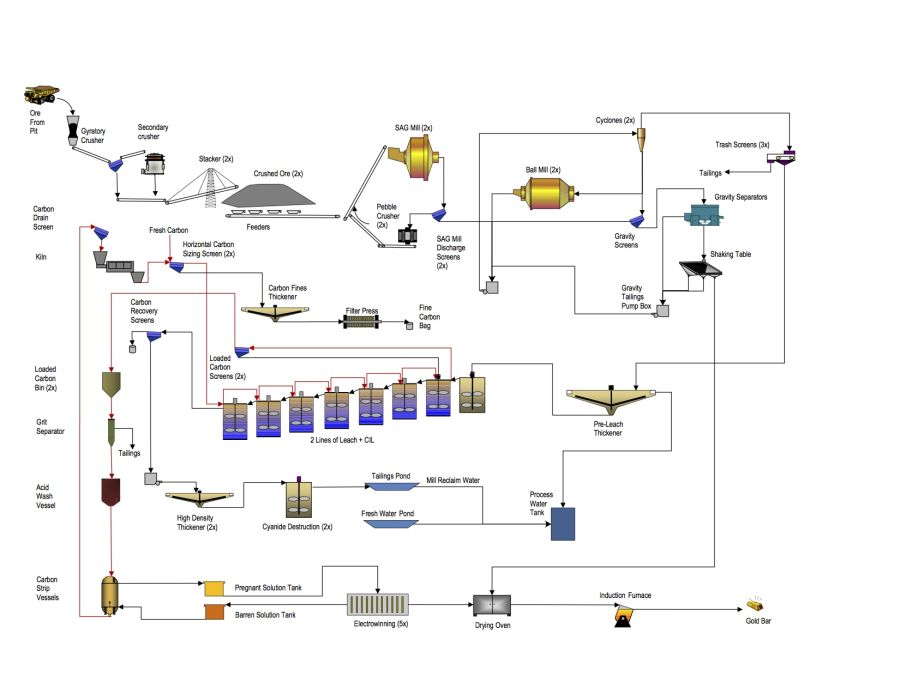
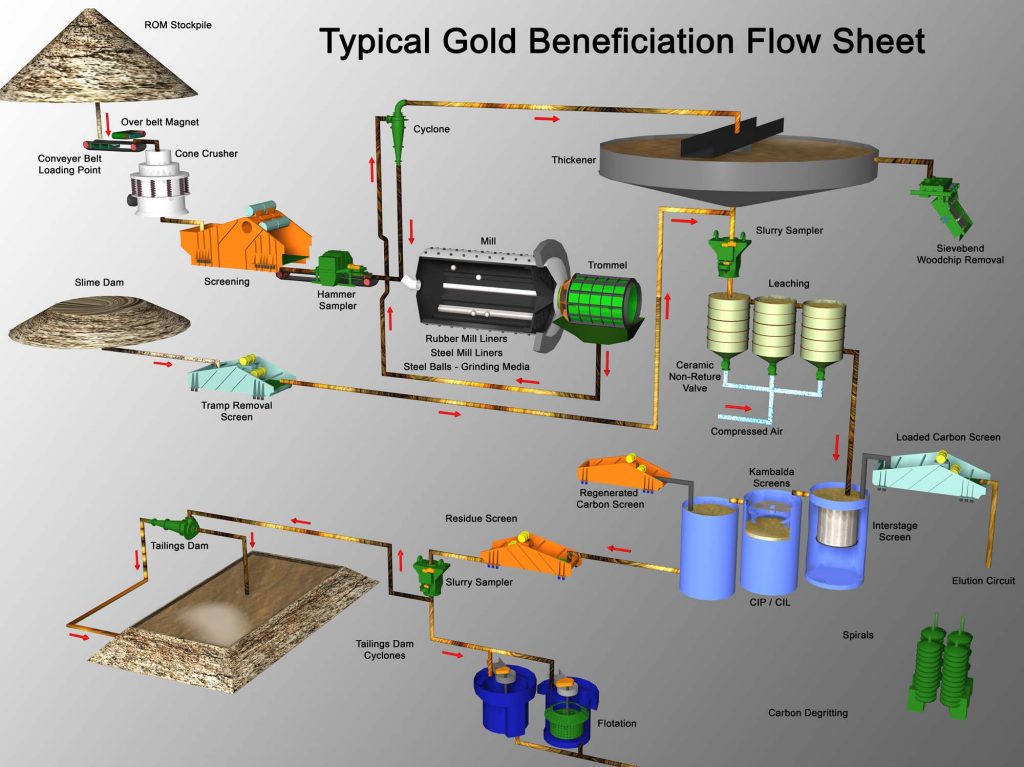
Gold and Silver Processing
- Grinding and Size Classification.
- Leaching and Adsorption:
- Addition of water to form slurry .
- Addition of lime to the ore and cyanide solution to the slurry, to leach the gold or silver.
- Addition of carbon to adsorb dissolved metals.
- Recovery and Dore Bullion
- Stripping the metals from the carbon by acid washing .
- Precipitation of the gold and silver by electro-wining.
- Smelting of metal products into bars of dore bullion.
- Pumping of the barren slurry (tailings) to the tailings storage facility.

[image 135-1-14.1]

[image 135-1-15]
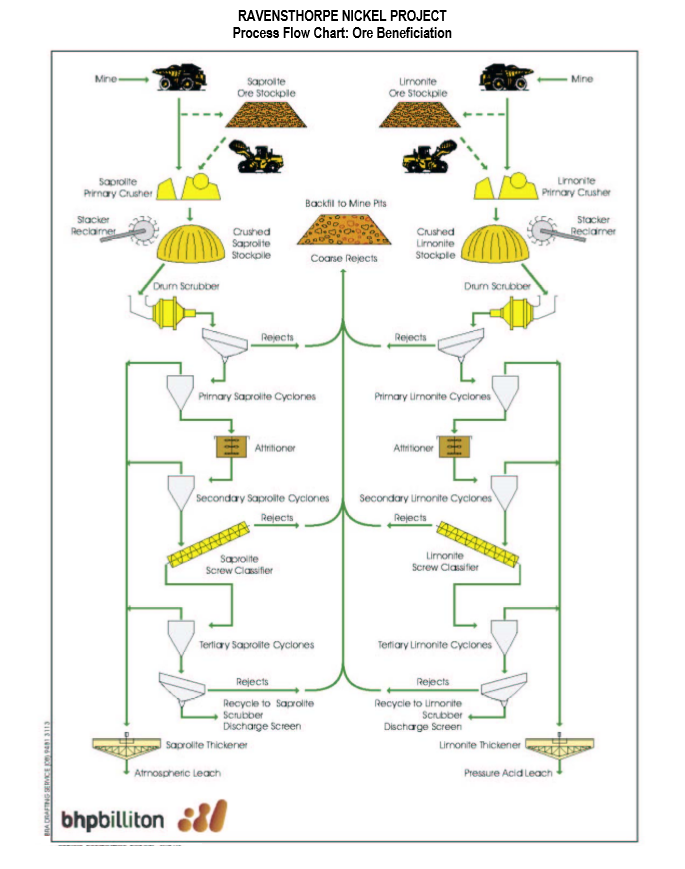
Nickel Processing
- Size Reduction: Primary, secondary and tertiary crushers.
- Magnetic Separation: Separates magnetic ore (pyrrhotite) from non-magnetic ore (copper and nickel concentrates).
- Froth Flotation: Non-magnetic ore is sent to a series of rougher and cleaner flotation cells to produce nickel concentrate.
- Drying: Thermal removal of liquid moisture .
- Calcining: Thermal decomposition of a material.
Nickel Processing Circuits
- Roasting: Thermal gas-solid reactions, which can include oxidation, reduction, chlorination, sulfation, and pyrohydrolysis.
- Smelting: Thermal reactions in which at least one product is a molten phase.
- Refining: Removal of impurities from materials by a thermal process .

[image 135-1-18.1]
[image 135-1-18.2]
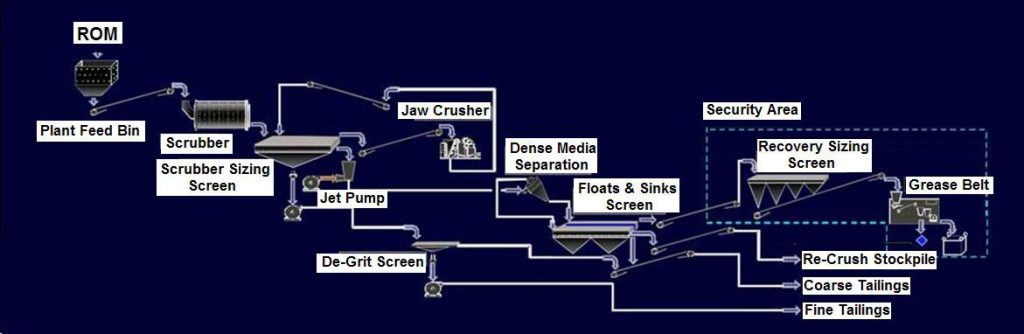
Diamond Processing
- Crushing
- Screening
- Heavy-Medium Separation (HMS)
- X-ray Sorter

[Image 135-1-19.1]


[image 135-1-19.3]

[image 135-1-19.4]
Uranium Processing
- Crushing and Grinding Circuit: Particle size reduction for leaching efficiency.
- Thickener: Removal of excess water from ground ore.
- Leaching: Acid is used to dissolve uranium from the ore.
- Washing and Filtering: Separation of uranium solution from solid waste.
- Solvent Extraction and Strip Section: Removal of uranium from water kerosene solution to aqueous solution.
- Precipitation Tanks: Uranium precipitates (yellowcake) upon addition of ammonia.
- Thickener: Excess water is removed.
- Centrifuge: Moisture removal.
- Calciner: Removal of ammonia and production of uranium oxide (U3O8).

[Image 135-1-21]
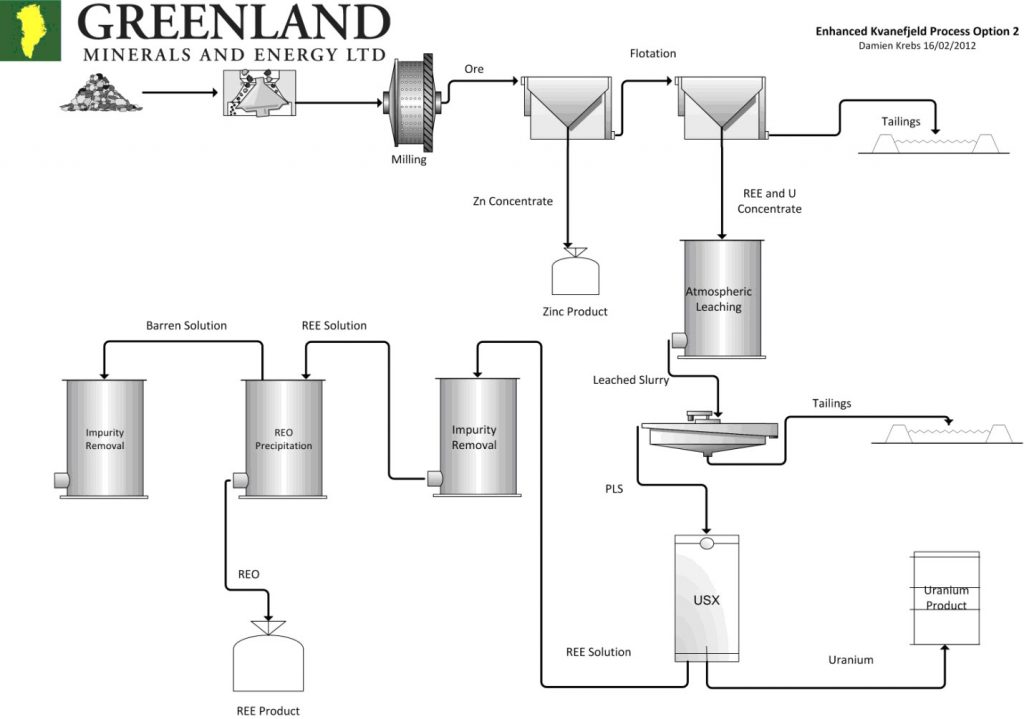
[Image 135-1-22]
Platinum Processing
- Size reduction: Crushing and milling circuits
- Rougher and Cleaner Flotation: Separation of platinum from ore
- Tailings and Concentrate Thickening: Removal of excess water.
- Filtering: Removal of moisture.
Platinum Processing Circuits
Smelting:
- Drying: Thermal removal of water
- Smelting : Thermal reactions in which at least one product is a molten phase
Refining:
- Base Metals Refinery: Nickel, copper and cobalt
- Platinum Metals Refinery: Platinum, Rhodium, Iridium, Ruthenium, Palladium and gold
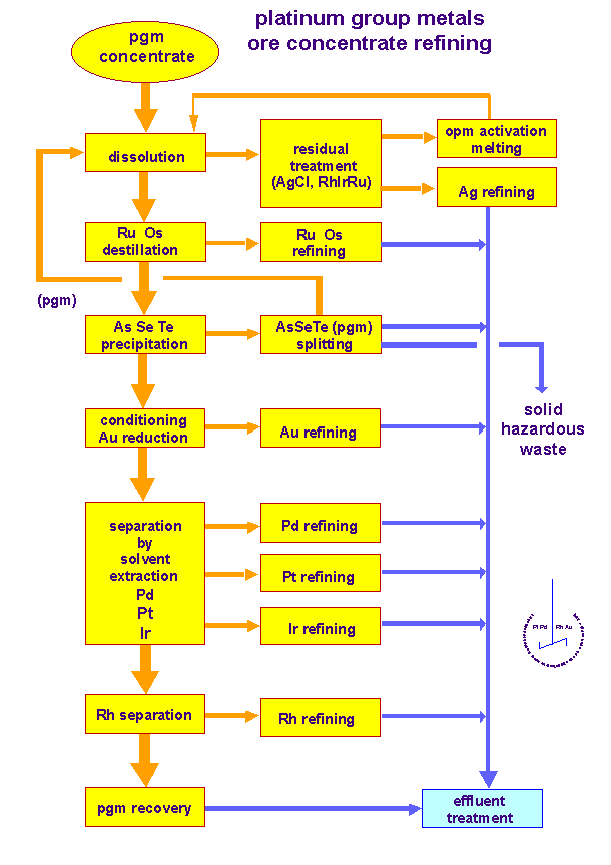
[image 135-1-24]
Zinc Processing Circuit
- Size reduction: Crushing and grinding.
- Flotation: Zinc is separated from the ore.
- Filtering: Removal of excess water and surface.

[image 135-1-25]

[image 135-1-25.2]

[image 135-1-25.3]
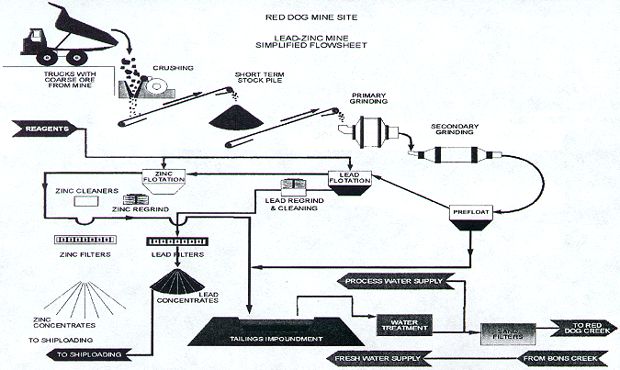
[image 135-1-26]
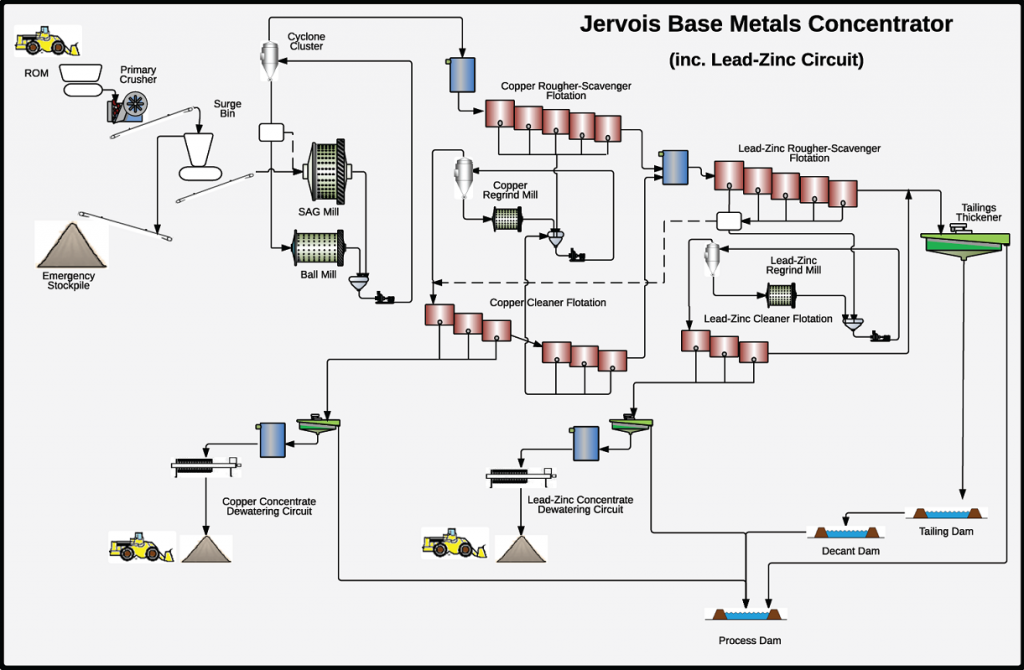
[image 135-1-27]
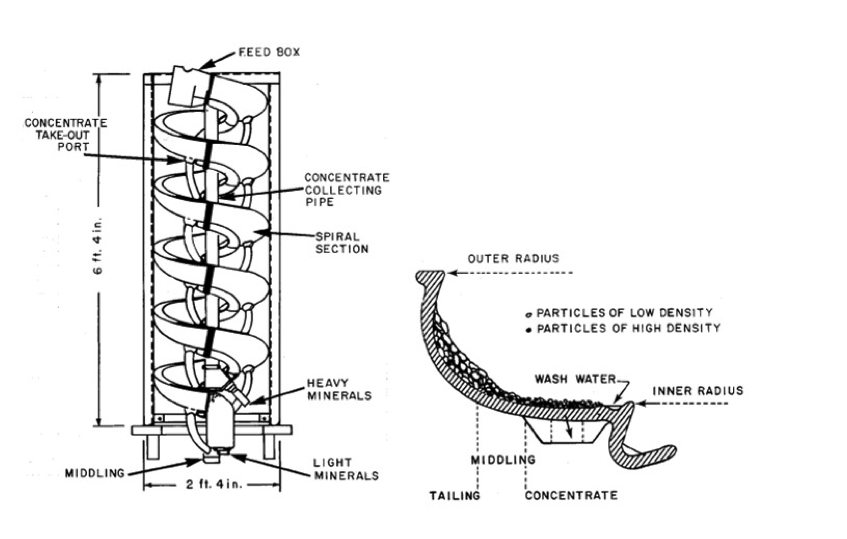
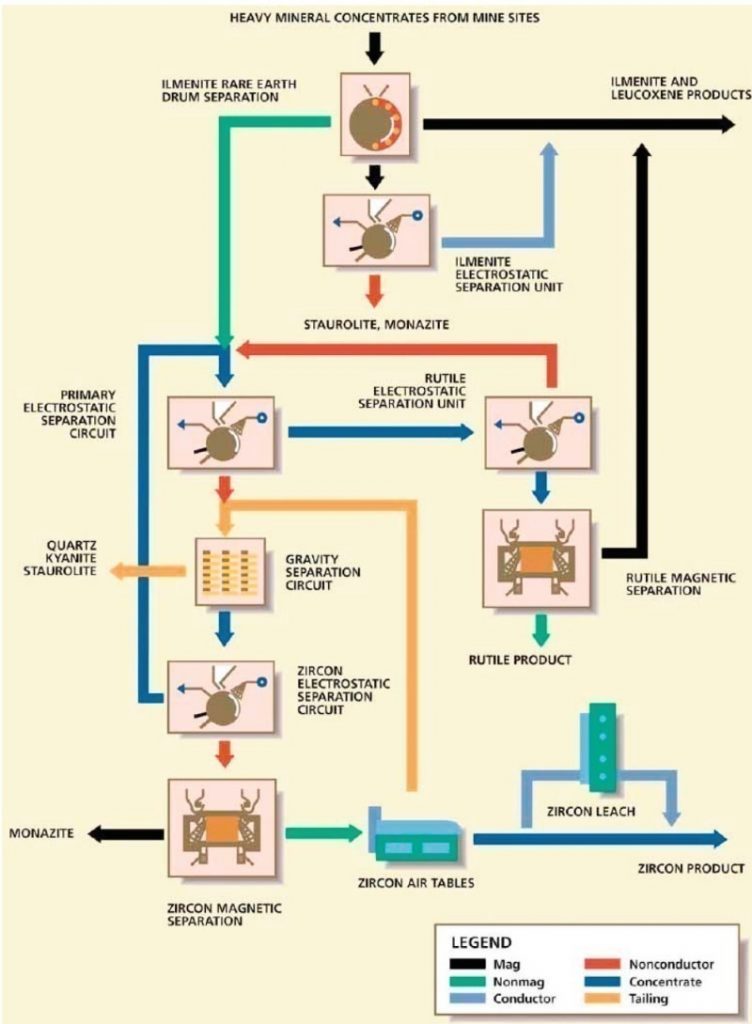
Heavy Mineral Sand
- Main products of heavy mineral sands processing are:
- Rutile (TiO2)
- Ilmenite (FeTiO3)
- Leucoxene
- Zircon (ZrSiO4)
- Titanium Dioxides
- Paints
- Plastics
- Paper
- Textiles
- Inks
- Foodstuffs, cosmetics

[image 135-1-28.1]

[image 135-1-28.2]
Zirconium
- Zircon is generally a minor product obtained from processing heavy mineral sands.
- Applications
- Ceramics in opacifiers used in surface glazes and pigments.
- High melting point (2200 °C) attracts use as a foundry sand in moulds
- Zirconium metal
- 90% in nuclear energy
- Zirconium Metal
- Zirconium chemicals

[image 135-1-29]

[image 135-1-29]
Heavy Mineral Sand Properties
| Mineral | Valuable | Magnetic Susceptibility | Electrical Conductivity | SG | Chemical Formula |
|---|---|---|---|---|---|
| Ilmenite | Yes | High | High | 4.5-5.0 | FeTiO3 |
| Rutile | Yes | Low | High | 4.2-4.3 | TiO2 |
| Zircon | Yes | Low | Low | 4.7 | ZrSiO4 |
| Leucoxene | Yes | Semi | High | 3.5-4.1 | Fe.TiO3.TiO2 |
| Monazite | No | Semi | Low | 4.9-5.3 | (Ce,La,Th,Nd,Y)PO4 |
| Staurolite | No | Semi | Low | 3.6-3.8 | Fe2Al9Si4O22.(OH) |
| Kyanite | No | Low | Low | 3.6-3.7 | Al2SiO5 |
| Garnet | No | Semi | Low | 3.4-4.2 | (Fe,MN,Ca)3.Al2(SiO4)3 |
| Quartz | No | Low | Low | 2.7 | SiO2 |
Magnetic & Non-Magnetic Density Fractionation
| Magnetic | Non-Magnetic | ||
|---|---|---|---|
| SG | Mineral | SG | Mineral |
| -3.85 | Trash | -3.79 | Quartz, trash |
| -3.85 + 4.05 | Magnetic Leucoxene | -3.79 + 4.05 | Leocoxene |
| -4.05 + 4.38 | Altered Ilmenite | -4.05 + 4.38 | Rutile |
| -4.38 + 4.9 | Primary Ilmenite | -4.38 + 4.9 | Zircon |
| +4.9 | Monazite | +4.9 | |



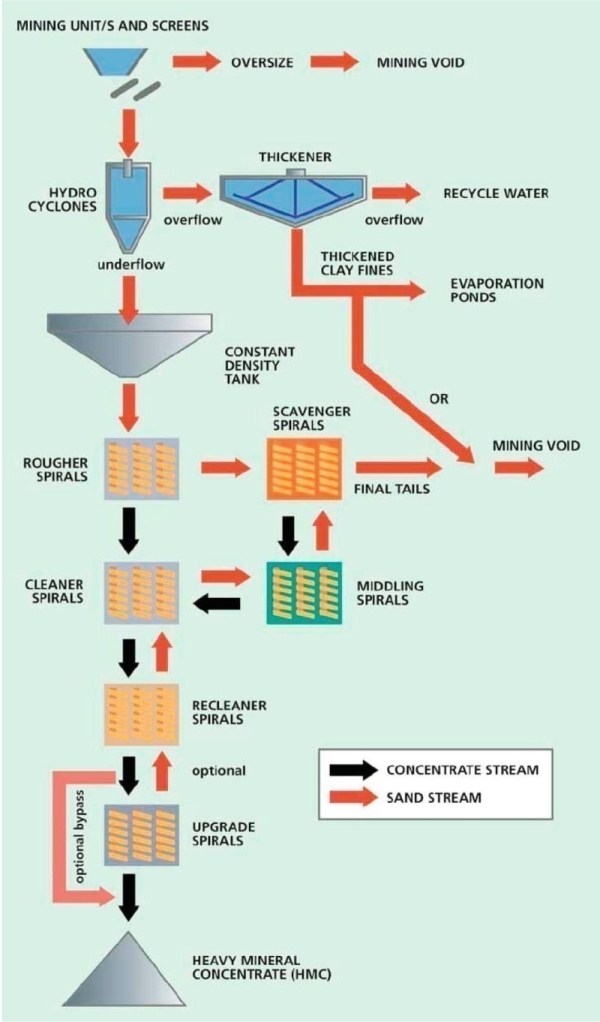
Wet High Intensity Magnets (WHIMS)
After the initial gravity concentration, WHIMS can be used to separate ilmenite from the other heavy minerals.

[image 136-1-36]