Contents
Objectives
- Discuss and identify major analytical instruments
- Discuss miscellaneous/specialty instruments
- Activity — PID/Instrument ID
- Test review
Reading
Terms to Know and Discuss
- Hand-Held, In-Line
- Visual, photometric
- pH, ORP, Conductivity
- Opacity, Turbidity
- Chromatograph, Spectrometer
- CEMS, personnel monitors
- Quantitative, Qualitative
- Rectilinear speed, Rotational speed
Why Monitor Analytical Variables?
- Environmental Monitoring/Reporting
- Mechanical integrity of Fixed Equipment
- Economics
- Product Quality Assurance
Which reason do you think is most important?
CEMS
- Continuous Environmental Monitoring Systems
- Reports emissions for EPA guidelines
- Sometimes on stacks, emissions from fired equipment
- Other systems required by regulation
- Mechanical Integrity
- Monitor corrosion rates
- Monitor corrosive atmospheres/liquids
- Monitor vibration, other indicators for rotating equipment
Economics
- Find problems real-time
- Correct problems real-time
- Save money
Product Quality
- Test finished products
- Test production streams
- May be dozens of parameters on products like fuels, chemicals
Qualitative vs. Quantitative
Qualitative
- To trigger response
- To make conversation
- Normal discussions
Quantitative
- To analyze in detail
- To make process corrections
Examples
- Baby, It’s cold outside (qualitative)
- It’s -47 °F outside (quantitative)
- There is benzene present in the air in the lab (qualitative)
- There is 0.5 ppb benzene present in the air in the lab (quantitative)
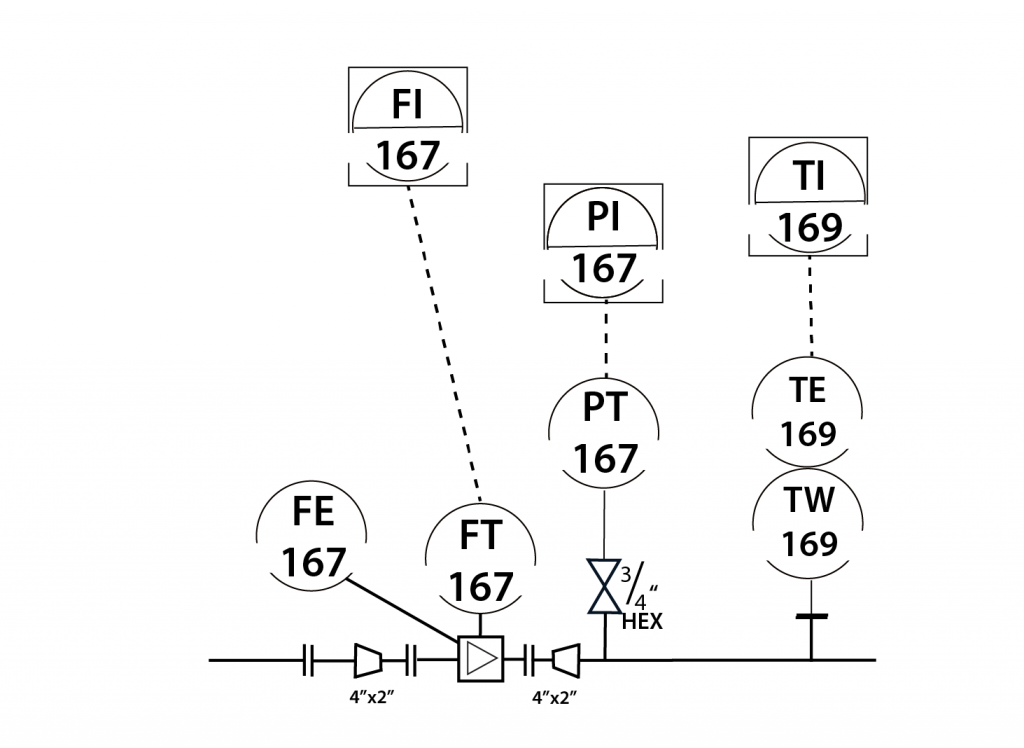
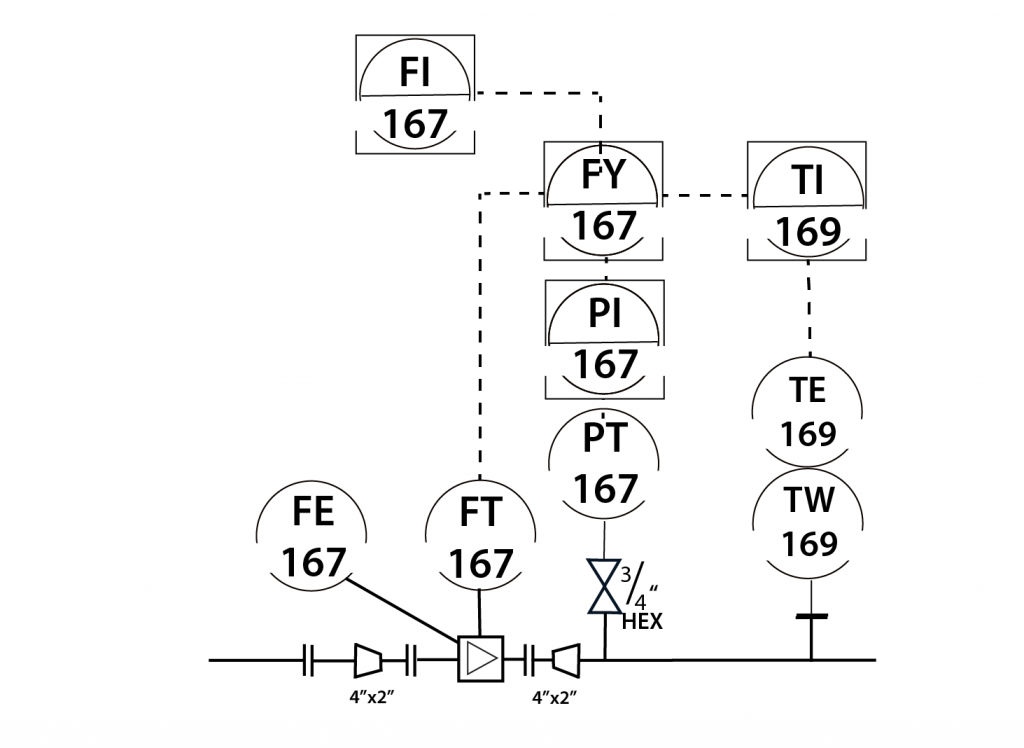
Sampling System
- Obtain a representative sample from the process stream.
- Transport the sample to the analyzer while maintaining its physical/chemical integrity.
- Analyze the sample.
- Return the sample to the process or discard it appropriately.
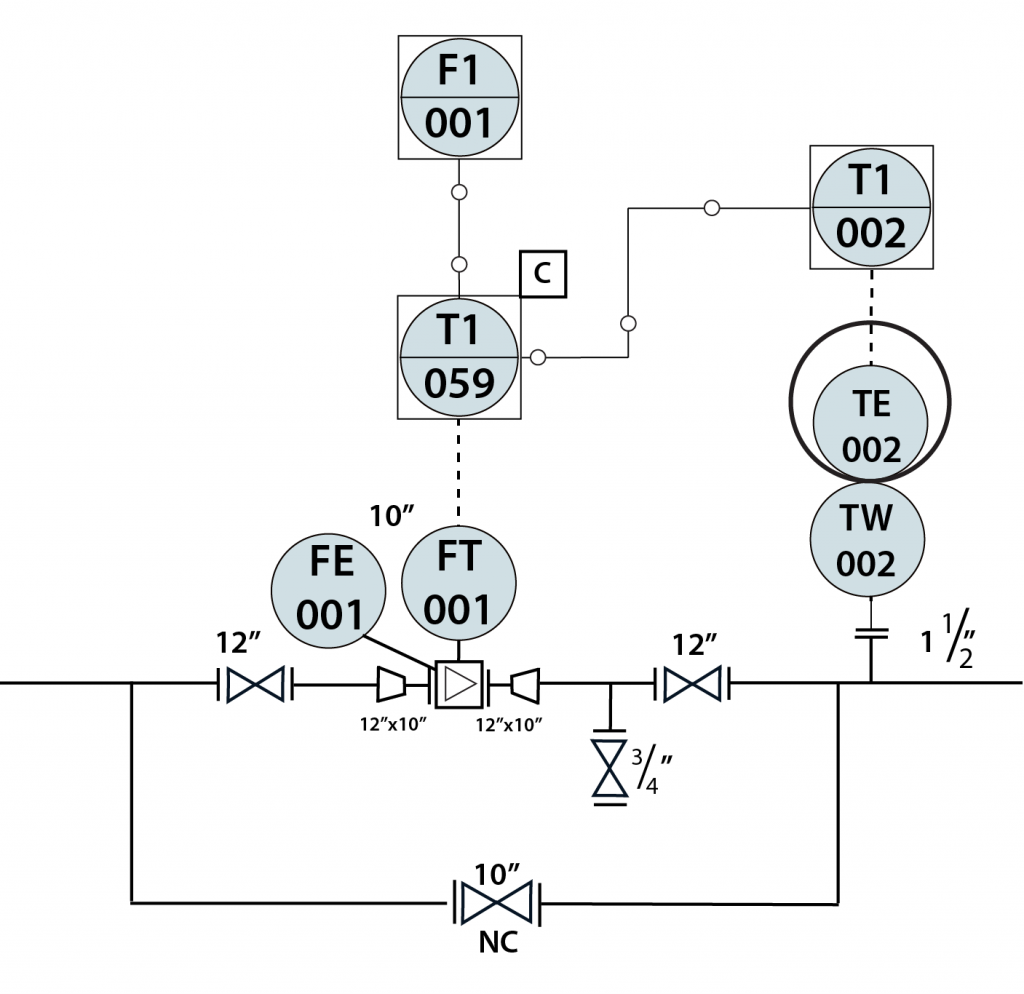
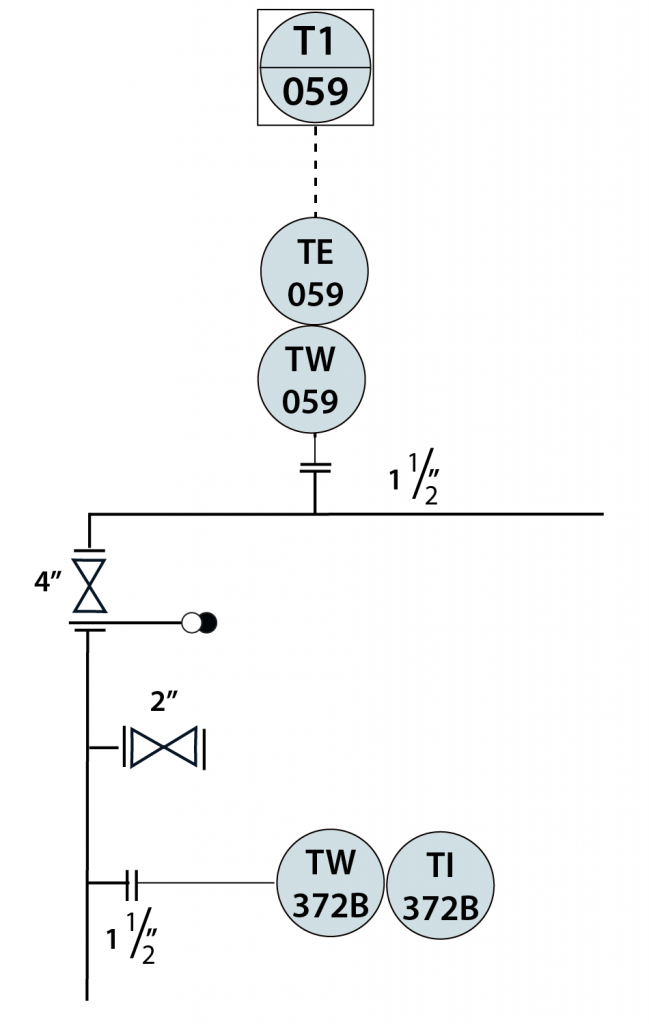
In-Line Instrument
- In-Line instruments are permanently installed within the process unit.
- The data from in-line instruments is used in product quality control, and/or environmental reporting.
Personal Monitor

[image-140-7-3] By Elfabriciodelamancha (Own work) [GFDL (https://www.gnu.org/copyleft/fdl.html) or CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Employee wears dosimeter during regular work for required time period, then it is sent away for analysis —
Determines TWA, STEL readings —
QUANTITATIVE ANALYSIS
Lab Instrument

[image: 140-7-4] By Mirolka (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
pH
pH — acidity measurement — hydrogen ions specifically
- Water systems and processes
- Indications of corrosion, scaling issues
- Scale = 0 to 14
- <7 = Acid
- >7 = Base (caustic)
- 7 = neutral
ORP — Oxidation-Reduction Potential
- Ratio of reducing agents to oxidizing agents in the sample
- Free electron concentration
- Similar to acid/base analysis
Chromatography
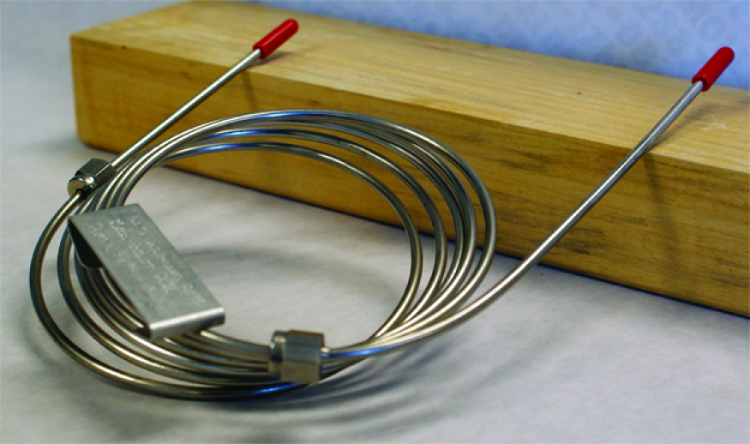
[image 140-7-5] Image from LibrTexts.org
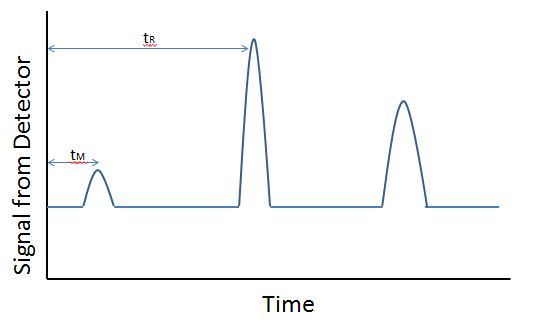
[image 140-7-6]
- Chromatography separates mixtures into components by forcing them through a ‘packed column’.
- Gas flows through column, pushes material through — heavier molecules take longer to move through
- As stream exits the column, different types of detectors used to read amount of material.
- Data = time through column, size of peak on chart
- Time through column = identify component
- Size of peak on chart = amount of component
- Use calibration standards to identify and quantify
- Other methods of separation — ability to adsorb onto column, polarity, etc — same principles
| pH meter | A | Measures the amount of particulate matter in a gas stream by measuring the transmittance or absorption of light through the material |
| ORP meter | B | Measures the ratio of reducing agents to oxidizing agents |
| Conductivity meter | C | Separates the molecular components of a liquid or gas by forcing them through a packed column. |
| Chromatograph | D | Measures the hydrogen ion concentration |
| Turbidity analyzer | E | Measures the ability of a solution to conduct electricity |
| Opacity analyzer | F | Measures the amount of particulate matter in a liquid stream by measuring the amount of transmittance or absorption of light through the material |
Vibration Monitors
- Why is it important to monitor vibration on rotating equipment?
- Excessive vibration is a sign that equipment is out of alignment
- Excessive vibration is a sign that equipment is wearing out — could fail
Rotation/Speed monitors
- Rectilinear = speed in a straight line — velocity
- i.e. meters/second, feet/sec
- Rotational = speed of revolution, for rotating equipment like pumps, motors
- i.e rpm